Abstract
Background
Patients with cancer have an increased risk of venous thromboembolism (VTE) and associated morbidity and mortality. Renal dysfunction is more common in patients with cancer, leading to heightened risks of bleeding and thrombotic complications. In the AVERT trial, thromboprophylaxis with apixaban resulted in a significantly lower rate of VTE and higher rate of major bleeding compared to placebo among intermediate-to-high-risk ambulatory cancer patients starting chemotherapy. As apixaban depends on some degree of renal clearance, there may be concerns regarding the safety and efficacy of apixaban thromboprophylaxis in patients with renal insufficiency. In this post-hoc analysis of AVERT, we evaluated the efficacy and safety of apixaban thromboprophylaxis according to renal function at randomization.
Methods
Eligible patients were randomized to apixaban (2.5mg twice daily) or placebo. First dose of study drug was given within 24 hours of the first chemotherapy administration with the intended treatment period of 180 days. For this subgroup analysis, the efficacy and safety of apixaban thromboprophylaxis was evaluated accordingly to renal function (calculated creatinine clearance [CrCl] by Cockcroft-Gault Equation) at randomization. Patients with CrCl < 30 mL/min were excluded from the trial. The primary efficacy outcome was objectively confirmed major VTE (proximal deep vein thrombosis or pulmonary embolism) within 180 days (±3 days) following randomization. The primary efficacy outcome was evaluated by modified intention-to-treat analysis, which included all patients who had undergone randomization and received at least one dose of study medication on or before day 180 (±3 days). The primary safety outcome was major bleeding defined by the International Society on Thrombosis and Haemostasis criteria. The primary safety outcome was evaluated by on-treatment analysis, when events were counted only if they occurred on study drugs or up to two days after discontinuation of the study drugs. Secondary outcomes included clinically relevant non-major bleeding and overall mortality.
Results
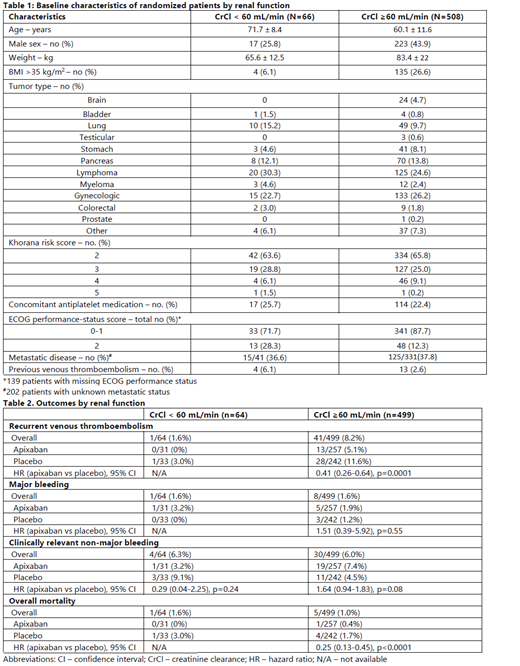
A total of 574 patients underwent randomization, with 563 patients included in the original primary efficacy and safety analysis (288 apixaban and 275 placebo). Upon randomization, 66 (11.5%) patients had CrCl < 60 mL/min and 508 (88.5%) patients had CrCl ≥ 60 mL/min. Patients with CrCl < 60 mL/min were significantly older, more female, had lower weight and fewer with body mass index (BMI) > 35 kg/m 2 and poorer ECOG performance status (Table 1). In patients with CrCl < 60 mL/min, VTE occurred in no patient on apixaban compared to 1 on placebo, and major bleeding episode occurred in 1 on apixaban and 0 on placebo. In patients with CrCl ≥ 60 mL/min, VTE occurred in 13 out of 257 (5.1%) in the apixaban group and 28 out of 242 (11.6%) in the placebo group [HR 0.41 (95% CI 0.26-0.64), p=0.0001] (Table 2). There were no significant differences between apixaban and placebo groups in major bleeding and clinically relevant non-major bleeding events. Overall mortality was significantly lower in the apixaban group (HR 0.25 [95% CI 0.13-0.45], p<0.0001) in patients with CrCl ≥ 60 mL/min.
Conclusions
In the AVERT trial, patients with CrCl < 60 mL/min were significantly older, more likely to be female, with lower weight or BMI and poorer ECOG performance status. There were very few VTE or major bleeding events in patients with CrCl < 60 mL/min. In patients with CrCl ≥ 60 mL/min, apixaban thromboprophylaxis was associated with a significantly lower rate of VTE and overall mortality compared to placebo, with no significant differences in the rates of major bleeding or clinically relevant non-major bleeding events.
Wang: Servier: Membership on an entity's Board of Directors or advisory committees; Leo Pharma: Research Funding. Carrier: Leo Pharma: Honoraria, Research Funding; BMS: Honoraria, Research Funding; Sanofi: Honoraria; Servier: Honoraria; Pfizer: Honoraria, Research Funding; Bayer: Honoraria. Wells: Daiichi Sankyo: Honoraria; BMS/Pfizer: Honoraria, Research Funding; Bayer: Honoraria; Sanofi: Honoraria.
apixaban for primary thromboporphylaxis in ambulatory cancer patients receiving chemotherapy